Silicon is an element – atomic number 14 – with an atomic weight of 28.0855. Silicon sits in the periodic table between the metal, aluminium, and the non-metal, phosphorus.
Silicon is a metalloid, which is a chemical element that possesses properties in between metals and non-metals or a mixture of metal and non-metal. However, there is no complete agreement or even a standard definition of a metalloid. There is also an argument of which elements are, in fact, metalloids.

A piece of pure silicon. The metalloid is one of the Earth’s most abundant elements and an important industrial material, used extensively in the electronics and energy sectors.
Photograph by Hi-Res Images of Chemical Elements
Metalloids, like silicon, typically have a metallic appearance but are too brittle to use in construction or engineering on their own.
Discovery of silicon
Silicon was isolated in 1823 by the Swedish chemist, Baron Jöns Jacob Berzelius. Berzelius was born on 20th August 1779 in Väversunda, Sweden and died on the 7th August 1848 in Stockholm.
Berzelius was a prolific chemist and went on to discover other elements, including thorium, cerium and selenium. His students identified lithium and vanadium. He was ennobled in 1818 by King Carl XIV Johan but was known as Jacob Berzelius throughout his life.

A statue of Jöns Jacob Berzelius, one of the founders of modern chemistry, in Berzelii Park, Stockholm. Berzelius Day is celebrated on 20th August each year in Sweden.
Photograph by Jim Henderson
Abundant element
Silicon is calculated to be the 8th most abundant element in the known universe. There is a current biological theory that postulates that silicon might even be the basis for other types of life on alien planets, rather than carbon.
Silicon is forged during the death throes of a star. At the very end of the star’s life, once all of its fuel is used, the star explodes in an enormous cosmic blast of energy. All the silicon that is in the star is then blasted outwards and can be utilised by cosmic processes to form rocky planets, like Earth.
On our planet, every time you hit the beach or fly over the Sahara desert, you see vast amounts of silicon dioxide also known as silica. It is the major constituent of sand.
Human applications of silicon
Silicon has been used for many centuries by humans in many ways. It is the main element of flint, which made the stone age possible and gave us axes and arrowheads.
In the information age, silicon is in everything from glass to computer chips. We use it in thousands of products, but do not often give the source of the material any thought. It is a semiconductor that has made desktop and laptop computers possible, and the average mobile phone’s total weight is made up of around 6% silicon.
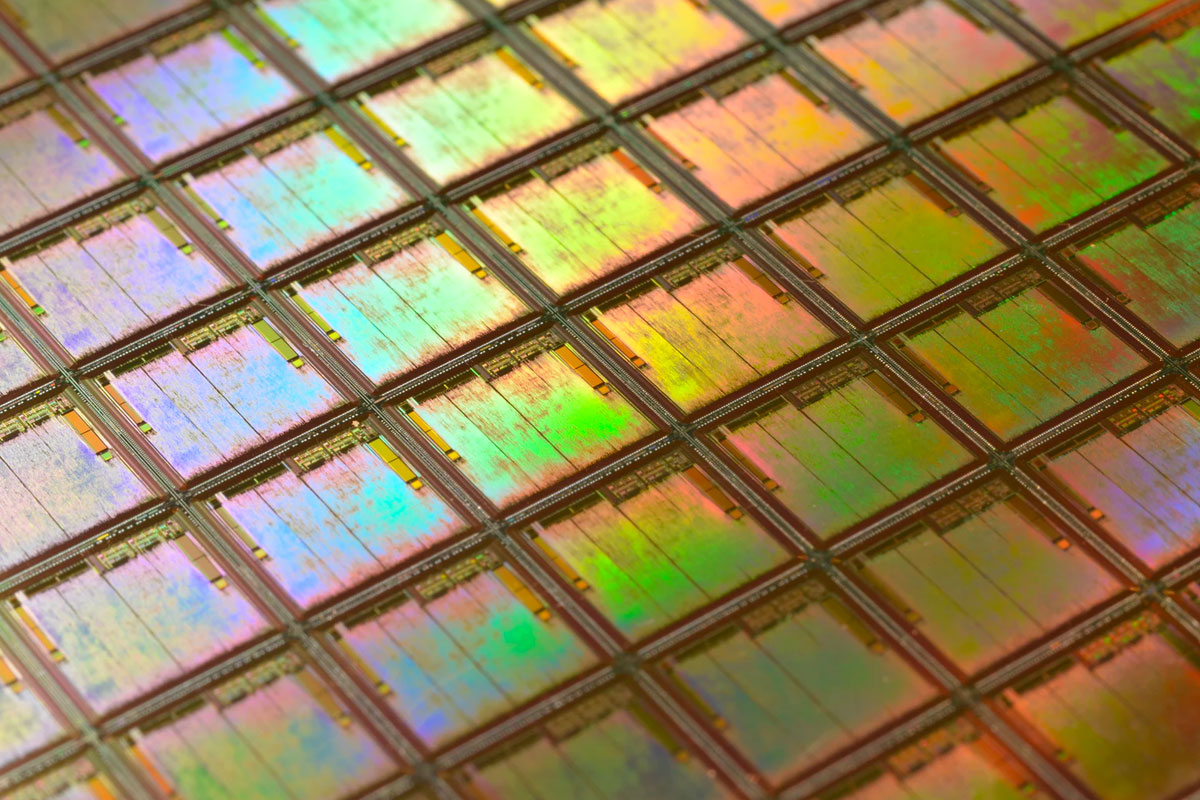
Close-up shot of a silicon wafer. Each square is a chip with microscopic transistors and circuits which goes into the processors that power our phones and computers.
Silicon alloyed with stainless steel
We also use small amounts of silicon for the production of stainless steel. Silicon is added to austenitic or non-magnetic stainless steel formulations to improve corrosion resistance to sulfuric acid.
The addition of silicon also helps to improve stainless steel’s oxidation resistance and is a ferrite stabilizer. In magnetic or austenitic stainless steels, a higher percentage of silicon helps to strengthen the stainless steel’s resistance to oxidation.
Applying heat and pressure to silicon gives us a ceramic material we call silicon carbide. It possesses an outstanding hardness.that can only be surpassed by diamond, cubic boron nitride and boron carbide. Silicon carbide is a far harder product than any stainless steel surface and is an ideal product for finishing metals of all types. We use silicon carbide to apply finishes to stainless steel coils and sheets for example Brush Number 4 and Hairline.

Porsche Carrera GT’s silicon carbide brake disc. This carbon-silicon composite is able to withstand extreme temperatures generated by high performance cars when braking.
Sheets of stainless steel containing silicon, and polished using belts containing silicon carbide, are a stock product used in Double Stone Steel’s PVD process.